Photobiomodulation (PBM therapy) is an innovative red / near infrared light treatment that is used to stimulate cellular functions. It utilizes non-ionizing light sources, including lasers, light emitting diodes (LED), and/or broadband light, in the visible (400 – 700 nm) and near-infrared (700 – 1100 nm) parts of electromagnetic spectrum.
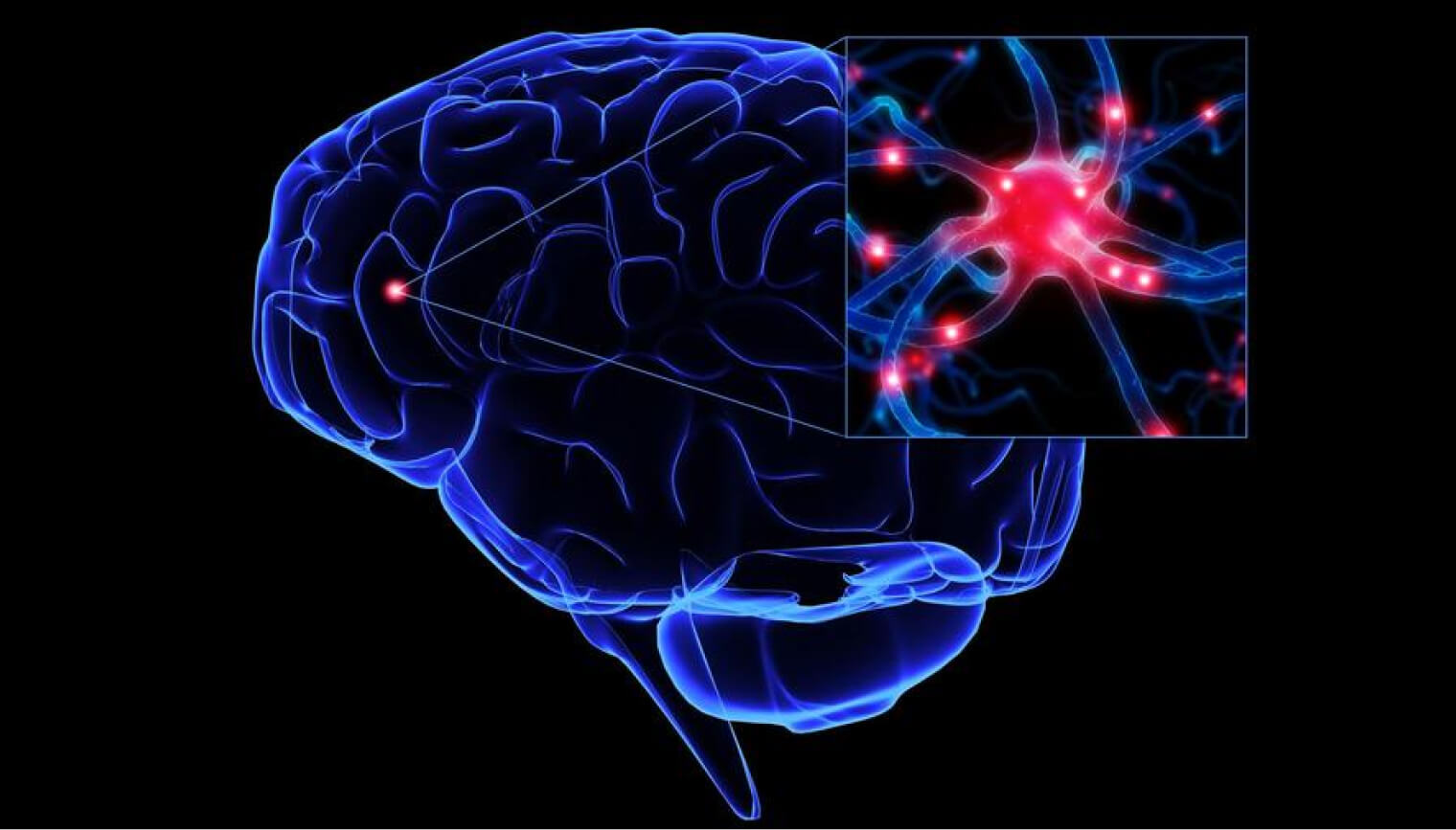
What is Photobiomodulation
How Does It Work
A light source is placed near or in contact with the skin, allowing the light energy (photons) to penetrate tissue. Light interacts with parts of cells, resulting in photophysical and photochemical changes that lead to alterations at the molecular, cellular and tissue levels of the body.
Effectiveness of PMB therapy depends on color of the light (wavelength), its intensity, the site of light application, total energy delivered, and other parameters of the treatment.

What is it Used For
Photobiomodulation is already used in multiple applications, and there are numerous pre-clinical and clinical studies exploring other areas. Some of the current uses of photobiomodulation include:
- Wound healing;
- Tissue regeneration;
- Reduction of inflammation;
- Reduction of pain;
- Restoration of normal cellular function;
- Hair Restoration;
- Skin rejuvenation.

Photobiomodulation and Neurological Disorders
Photobiomodulation is currently studied for multiple neurological disorders. In particular, PBM
- Has improved recovery in stroke patients;
- Has improved cognition of veterans with traumatic brain injury;
- Has slowed down dementia;
- Has reduced anxiety and PTSD;
- Is currently studied for the effect on persons with Down Syndrome, opioid addicts and other patients.
Photobiomodulation and Autism
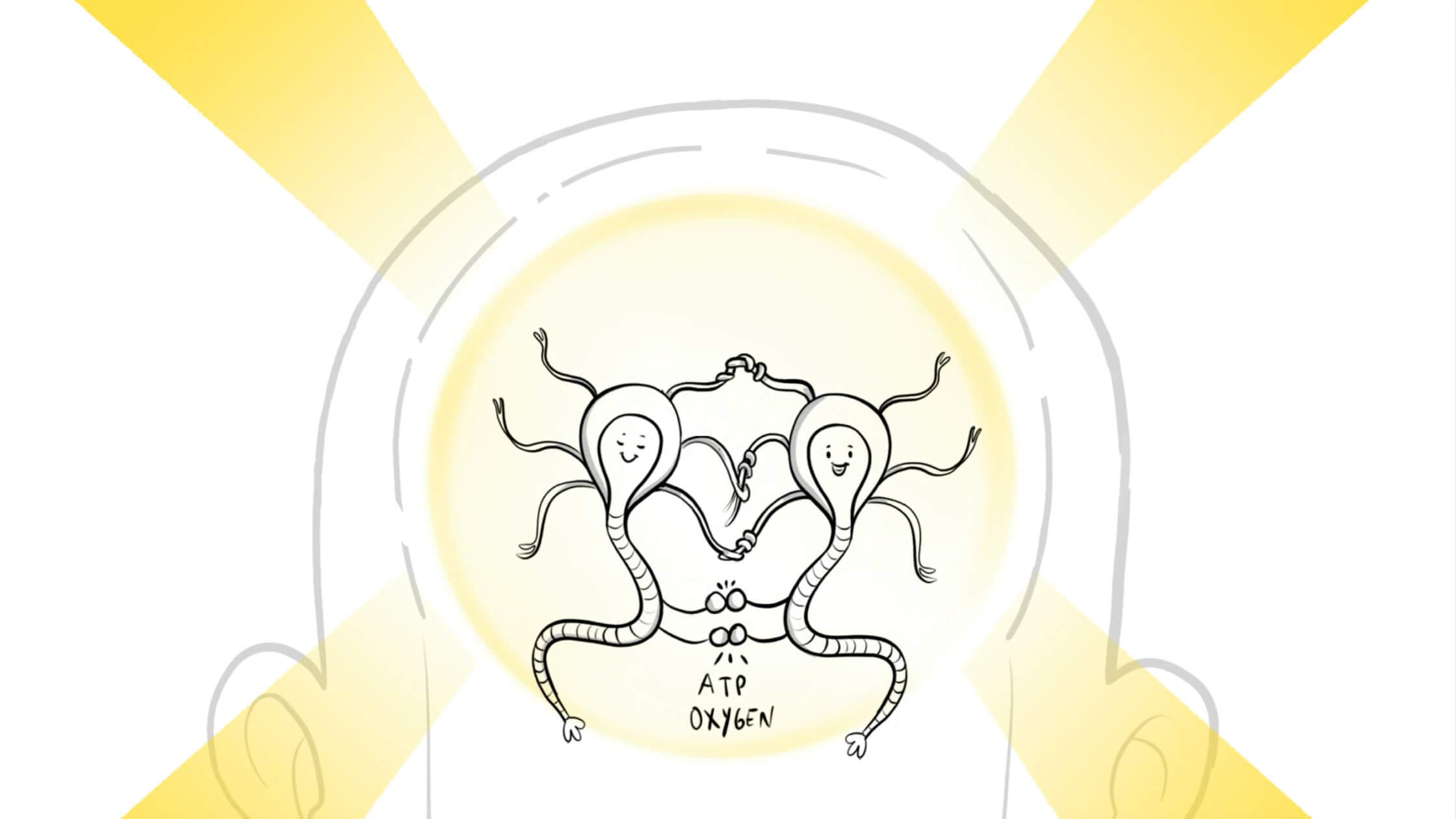
Why is it effective for autism
Recent neuroscience studies show correlation between Autism and Mitochondria Dysfunction.
- Mitochondria is the cellular power plant inside every cell.
- Individuals with autism frequently have mitochondria dysfunction where not sufficient amount of ATP is generated from food proteins.
- With photobiomodulation, Red and Near Infrared light penetrates the brain tissue and is absorbed by mitochondria in the cells.
- PBM therapy has a photochemical effect on the mitochondria. Mitochondria absorbs light photons, which leads to more ATP (cellular energy molecule) productions.
- More ATP results in more energy, tissue repair and anti-inflammatory effects.
- Simultaneous stimulating of targeted brain areas promotes functional brain connectivity
The result is the decrease of autism symptoms, increase in eye contact, increase in responsiveness, and speech improvement.
Photobiomodulation Theory
Hamblin, M. R (2016). Shining light on the head: Photobiomodulation for brain disorders. BBA Clinical, 6, 113-124.
Hamblin, M.R. (2019).Mechanisms of photobiomodulation in the brain. In MR Hamblin and YY Huang (eds). Photobiomodulation in Brain, 1st edition.
Johnson, D.M., Moro, C., Stone, J., Bhabid, A.L,, Mitrofanis, J.(2015). Turning On Lights to Stop Neurodegeneration: The Potential of Near Infrared Light Therapy in Alzheimer’s and Parkinson’s Disease. Frontiers in Neurscience, 9.
Naeser, M. A., and Hamblin, M.R. (2011). Potential for Transcranial Laser or LED Therapy to Treat Stroke, Traumatic Brain Injury, and Neurodegenerative Disease. Photomedicin and Laser Surgery. 29 (7), 443-446.
Naeser MA, Hamblin MR. (2015). Traumatic Brain Injury: A Major Medical Problem That Could Be Treated Using Transcranial, Red/Near-Infrared LED Photobiomodulation. Journal of Photomedicine and Laser Surgery. 33(9):443-6
Naeser MA, Martin PI, Ho MD, Krengel MH, Bogdanova Y, Knight JA, Yee MK, Zafonte R, Frazier J, Hamblin MR, Koo BB. (2016). Transcranial, Red/Near-Infrared Light-Emitting Diode Therapy to Improve Cognition in Chronic Traumatic Brain Injury. Journal of Photomedicine and Laser Surgery, 34(12):610-626
Saltmarche AE, Naeser MA, Ho KF, Hamblin MR, Lim L. (2017). Significant Improvement in Cognition in Mild to Moderately Severe Dementia Cases Treated with Transcranial Plus Intranasal Photobiomodulation: Case Series Report. Journal of Photomedicine and Laser Surgerym 35(8):432-441
Autism and Mitochondrial Disorder
Chalika, D, Singh, LN, Leipzig, J, Lvova, M, Derbeneva O, Lakatos A, Hadley D, Hakonarson H, Wallace DC (2017). Associations between Mitochondria and Autism Spectrum Disorder. JAMA Psychiatry, 74(11), 1161-1168.
Giulivi C1, Zhang YF, Omanska-Klusek A, Ross-Inta C, Wong S, Hertz-Picciotto I, Tassone F, Pessah IN. (2010). Mitochondrial dysfunction in autism. JAMA, 304(21):2389-96
Martinez – Pedraza, FL and Carter, A (2009). Autism spectrum Disorder in Young Children. Journal of Child and Adolescent Psychiatry, 18(3). 645-664.
Siddiqui, M.F, Elwel, C; Johnson, M.H.(2016). Mitochondria dysfunction in Autism Spectrum Disorder. Autism Open Access 6(5).
Valiente-Pallejà A, Torrell H, Muntané G, Cortés MJ, Martínez-Leal R, Abasolo N, Alonso Y, Vilella E, Martorell, L. (2018). Genetic and clinical evidence of mitochondrial dysfunction in autism spectrum disorder and intellectual disability. Human Molecular Genetics 27(5):891-900
Varga, N.A., Pentelenyj, K., Balisca, P, Gezsi, A, Remenyj, V, Harsfalvi, V., Bencsik, R. Illes, A., Prekop, C., Monar, M.J. (2018). Mitochondrial dysfunction and autism: comprehensive genetic analyses of children with autism and mtDNA deletion. Behavioral and Brain functions, 14 (4).
Wang, Y, Picard, M, Gu, Z (2016). Genetic Evidence for Elevated Pathogenicity of Mitochondrial DNA Heteroplasmy in Autism Spectrum Disorder. Public Library of Science: Genetics.
Weissman JR, Kelley RI, Bauman ML, Cohen BH, Murray KF, Mitchell RL, (2008). Mitochondrial Disease in Autism Spectrum Disorder Patients: A Cohort Analysis. Public Library of Science.
Autism and Mitochondrial Disorder
Leisman, G; Machado, C; Machado Y, Chinchilla-Acosta M (2018). Effects of Low-Level Laser Therapy in Autism Spectrum Disorder. Advances in Experimental Medicine and Biology, 1116:111-130.